抗生素抗性基因的传播机制及对策
DOI:
https://doi.org/10.52810/CJNS.2024.002Keywords:
抗生素抗性基因, 传播机制, 对策, 基因编辑, 人工智能Abstract
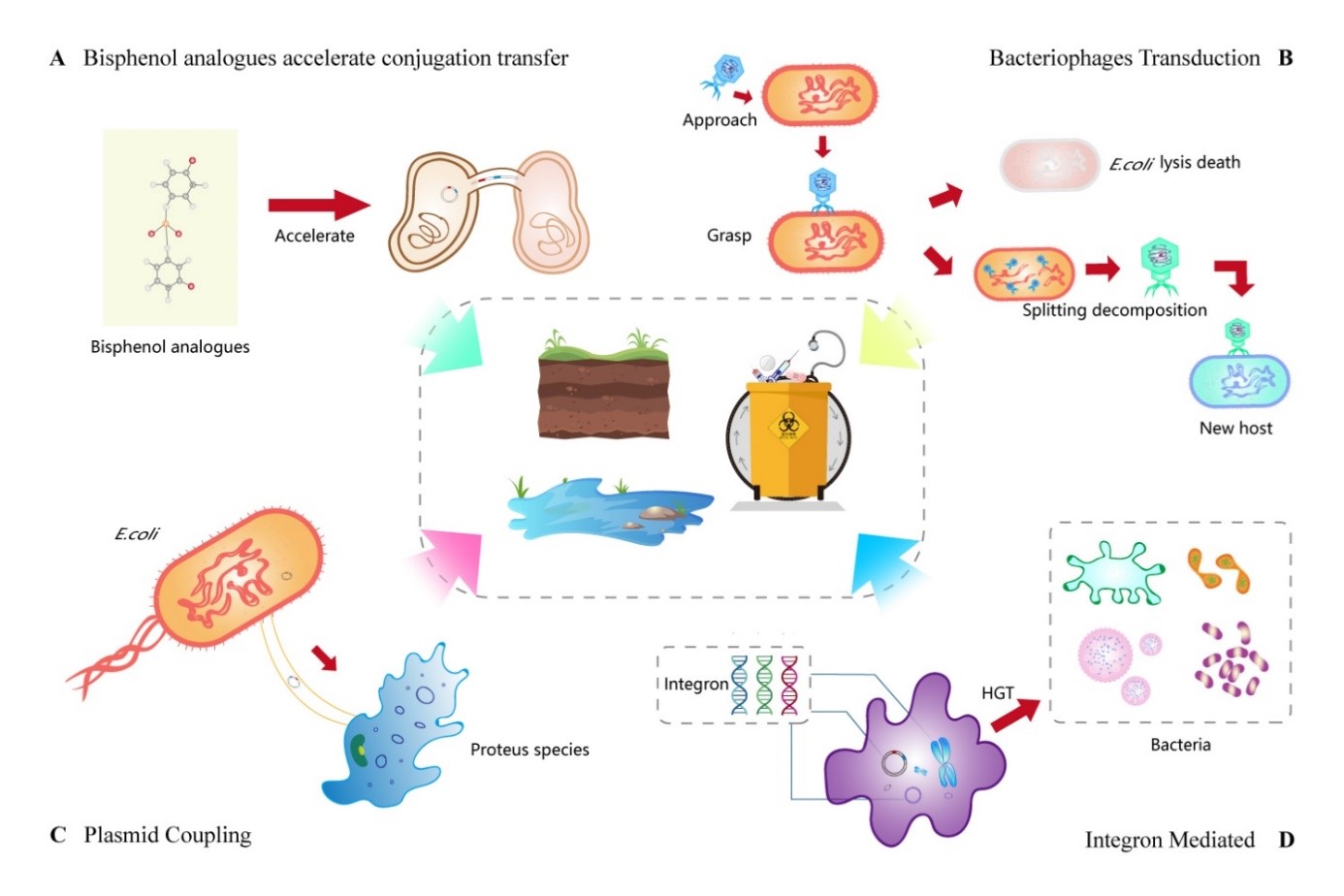
抗生素的出现极大地便利了生活,但由于滥用抗生素,全球抗药性的扩散对公共卫生构成了严重威胁。用于治疗和预防的抗生素正在全球范围内得到越来越广泛的使用。耐药菌株的数量在增加,越来越多的耐药基因正在出现。这将给自然环境、人类生产和生活带来更大的潜在危害。本文简要介绍了污水处理中抗生素抗性基因(ARGs) 传播的现状。此外,总结了基因编辑、全基因组测序 (WGS) 和人工智能 (AI) 在降低或阻止抗生素抗性扩散方面的应用,并探讨和展望了几种污水处理过程的工程可行性。这为找到能减少抗微生物药物抗性基因的出现,从根源上抑制抗生素抗性基因的传播,并最大限度地维护生活环境和保护公共健康安全的方法提供了参考。
References
Xu C, Kong L, Liao Y, et al. Mini-review: antibiotic-resistant Escherichia coli from farm animal-associated sources [J]. Antibiotics, 2022, 11(11): 1535-1552.
Balcazar J. How do bacteriophages promote antibiotic resistance in the environment? [J]. Clinical Microbiology And Infection, 2018, 24(5): 447-449.
Mutuku C, Gazdag Z, Melegh S. Occurrence of antibiotics and bacterial resistance genes in wastewater: resistance mechanisms and antimicrobial resistance control approaches [J]. World Journal Of Microbiology & Biotechnology, 2022, 38(9): 152-179.
Yang D, Wang J, Qiu Z, et al. Horizontal transfer of antibiotic resistance genes in a membrane bioreactor [J]. Journal of Biotechnology, 2013, 167(4): 441-417.
Sharma D, Misba L, Khan A. Antibiotics versus biofilm: an emerging battleground in microbial communities [J]. Antimicrobial Resistance and Infection Control, 2019, 8: 76-86.
Sugimoto S, Okuda K, Miyakawa R, et al. Imaging of bacterial multicellular behaviour in biofilms in liquid by atmospheric scanning electron microscopy [J]. Scientific Reports, 2016, 6: 25889-25902.
Cesare A, Fontaneto D, Doppelbauer J, et al. Fitness and recovery of bacterial communities and antibiotic resistance genes in urban wastewaters exposed to classical disinfection treatments [J]. Environmental Science & Technology, 2016, 50(18): 10153-10161.
Zhang S, Wang Y, Lu J, et al. Chlorine disinfection facilitates natural transformation through ROS-mediated oxidative stress[J]. The ISME Journal, 2021, 15(10): 2969-2985.
Wu J, Liu D, Li H, et al. Controlling pathogenic risks of water treatment biotechnologies at the source by genetic editing means [J]. Environmental Microbiology, 2021, 23(12): 7578-7590.
Tateno M, Umeyama T, Inukai T, et al. Examination of Cyp51A-resistance in aspergillus ientulus using CRISPR/Cas9 genome editing [J]. Medical Mycology, 2022, 63(2): 27-35.
Zhang S, Wang Y, Lu J, et al. Chlorine disinfection facilitates natural transformation through ROS-mediated oxidative stress [J]. ISME Journal, 2021, 15(10): 2969-2985.
Tao S, Chen H, Li N, et al. The application of the CRISPR-Cas system in antibiotic resistance [J]. Infection and Drug Resistance, 2022, 15: 4155-4168.
Dong H, Xiang H, Mu D, et al. Exploiting a conjugative CRISPR/Cas9 system to eliminate plasmid harbouring the mcr-1 gene from Escherichia coli [J]. International Journal of Antimicrobial Agents, 2019, 53(1): 1-8.
Wang P, He D, Li B, et al. Eliminating mcr-1-harbouring plasmids in clinical isolates using the CRISPR/Cas9 system [J]. Journal of Antimicrobial Chemotherapy, 2019, 74(9): 9-16.
Chen G, Cheng D, Chen B. Development of CRISPR technology and its application in bone and cartilage tissue engineering [J]. Nan Fang Yi Ke Da Xue Xue Bao, 2019, 39(12): 1515-1520.
Zou J, Tang Z, Yan J, et al. Dissemination of linezolid resistance through sex pheromone plasmid transfer inEnterococcus faecalis [J]. Frontiers in Microbiology, 2020, 11: 1185-1196.
Borges A, Castro B, GOVINDARAJAN S, et al. Bacterial alginate regulators and phage homologs repress CRISPR-Cas immunity [J]. Nature Microbiology, 2020, 5(5): 679-687.
Fage C, Lemire N, Moineau S. Delivery of CRISPR-Cas systems using phage-based vectors [J]. Current Opinion in Biotechnology, 2021, 68: 174-180.
Zhang H, Cheng Q, Liu A, et al. A novel and efficient method for bacteria genome editing employing both CRISPR/Cas9 and an antibiotic resistance cassette [J]. Frontiers in Microbiology, 2017, 8: 812-823.
Geng B, Huang X, Wu Y, et al. Identification and characterization of genes related to mmpicillin antibiotic resistance in zymomonas mobilis [J]. Antibiotics, 2022, 11(11): 1476-1488.
Oliveira M, Leonardo I C, Nunes M, et al. Environmental and pathogenic carbapenem resistant bacteria isolated from a wastewater treatment plant harbour distinct antibiotic resistance mechanisms[J]. Antibiotics, 2021, 10(9): 1118.
Easler M, Cheney C, Johnson D, et al. Resistome characterization of extended-spectrum beta-lactamase (ESBL)-producing Escherichia coli isolated from wastewater treatment utilities in Oregon [J]. Journal Of Water And Health, 2022, 20(4): 670-679.
Jiang X, Cui X, Liu W, et al. Genetic characterization of a novel sequence type of multidrug-resistant Citrobacter freundii strain recovered from wastewater treatment plant [J]. Infection and Drug Resistance, 2019, 12: 2775-2779.
Ingle D, Levine M, Kotloff K, et al. Dynamics of antimicrobial resistance in intestinal Escherichia coli from children in community settings in South Asia and sub-Saharan Africa [J]. Nature Microbiology, 2018, 3(9): 1063-1073.
Li Y, Van C, Boers S, et al. The diagnostic value of nasal microbiota and clinical parameters in a multi-parametric prediction model to differentiate bacterial versus viral infections in lower respiratory tract infections [J]. PLoS One, 2022, 17(4): 267140-267160.
Xu S, Yuan H. A three-methylation-driven gene-based deep learning model for tuberculosis diagnosis in patients with and without human immunodeficiency virus co-infection [J]. Microbiology And Immunology, 2022, 66(6): 317-323.
Sunuwar J, Azad R. Identification of novel antimicrobial resistance genes using machine learning, homology modeling, and molecular docking [J]. Microorganisms, 2022, 10(11): 2102-2117.
Schlessinger A, Espinoza J, Dupont C, et al. Predicting antimicrobial mechanism-of-action from transcriptomes: A generalizable explainable artificial intelligence approach [J]. Plos Computational Biology, 2021, 17(3): 1008857-1008882.
Lu J, Chen J, Huang L, et al. Rapid identification of species, antimicrobial-resistance genotypes and phenotypes of gram‐positive cocci using long short-term memory raman spectra methods [J]. Advanced Intelligent Systems, 2023, 10(1): 235-245.
Artini M, Patsilinakos A, Papa R, et al. Antimicrobial and antibiofilm activity and machine learning classification analysis of essential oils from different mediterranean plants against pseudomonas aeruginosa [J]. Molecules, 2018, 23(2): 482-495.
Patsilinakos A, Artini M, Papa R, et al. Machine learning analyses on data including essential oil chemical composition and in vitro experimental antibiofilm activities against staphylococcus species [J]. Molecules, 2019, 24(5): 890-916.
Freudenberg R, Wittemeier L, Einhaus A, et al. The spermidine synthase gene SPD1: a novel auxotrophic marker for chlamydomonas reinhardtii designed by gene editing [J]. Cells, 2022, 11(5): 837-854.
Gratacap R, Regan T, Dehler C, et al. Efficient CRISPR/Cas9 genome editing in a salmonid fish cell line using a lentivirus delivery system [J]. BMC Biotechnology, 2020, 20(1): 35-44.
Kim M, Kim H, Jeong D, et al. Cytosine base editor-mediated multiplex genome editing to accelerate discovery of novel antibiotics in bacillus subtilis and paenibacillus polymyxa [J]. Frontiers in Microbiology, 2021, 12: 691839-691851.
Minh-duy P, Peters K, Fraga L, et al. Plasmid-mediated ciprofloxacin resistance imparts a selective advantage on Escherichia coli ST131 [J]. Antimicrobial agents and chemotherapy, 2022, 66(1): 2146-2157.
Ruotsalainen P, Penttinen R, Mattila S, et al. Midbiotics: conjugative plasmids for genetic engineering of natural gut flora [J]. Gut Microbes, 2019, 10(6): 643-653.
Wu Z, Huang Y, Chao W, et al. Reversal of carbapenem-resistance in Shewanella algae by CRISPR/Cas9 genome editing [J]. Journal of Advanced Research, 2019, 18: 61-69.
Xu C, Liu H, Pan X, et al. Mechanisms for development of ciprofloxacin resistance in a clinical isolate of pseudomonas aeruginosa [J]. Frontiers in Microbiology, 2021, 11: 598291-5982919.
Xu Z, Li M, Li Y, et al. Native CRISPR-Cas-mediated genome editing enables dissecting and sensitizing clinical multidrug-resistant P.aeruginosa [J]. Cell Reports, 2019: 1707-1717.
Zhang N, He J, Muhammad A, et al. CRISPR/Cas9-mediated genome editing for pseudomonas fulva, a novel pseudomonas species with clinical, animal, and plant-associated isolates [J]. International Journal of Hygiene and Environmental Health, 2022, 23(10): 5443-5456.
Aksomaitiene J, Novoslavskij A, Kudirkiene E, et al. Whole genome sequence-based prediction of resistance determinants in high-level multidrug-resistant campylobacter jejuni Isolates inlithuania [J]. Microorganisms, 2021, 9(1): 66-77.
Fatahi-bafghi M, Naseri S, Alizehi A. Genome analysis of probiotic bacteria for antibiotic resistance genes [J]. Antonie Van Leeuwenhoek, 2022, 115(3): 375-389.
Kumar M, sodhi K, Singh D. Draft genome of Serratia sp. R1 gives an insight into the antibiotic resistant genes against multiple antibiotics [J]. Molecular Biology Reports, 2022, 49(6): 4479-4484.
Pouget C, Chatre C, Lavigne J, et al. Effect of antibiotic exposure on staphylococcus epidermidis responsible for catheter-related bacteremia [J]. International Journal Of Molecular Sciences, 2023, 24(2): 1547-1563.
Pouget C, Chatre C, Lavigne J, et al. Effect of antibiotic exposure on staphylococcus epidermidis responsible for catheter-related bacteremia [J]. International Journal Of Molecular Sciences, 2023, 24(2): 1547-1563.
Thach X, Trang L, Trieu L, et al. Whole-genome sequencing and characterization of an antibiotic resistant Neisseria meningitidis B isolate from a military unit in Vietnam [J]. Annals Of Clinical Microbiology And Antimicrobials 2019, 18(1): 16-25.
Turumtay H. Whole-genome sequencing-based characteristics of Escherichia coli Rize-53 isolate from Turkey [J]. Advances in Clinical and Experimental Medicine, 2022: 152704-152710.
Turumtay H, Allam M, Sandalli A, et al. Characteristics in the whole-genome sequence of Klebsiella pneumoniae ST147 from turkey [J]. Acta Ichthyologica Et Piscatoria, 2022, 69(2): 144-149.
Ullmann I, Nygaard A, Benteson, et al. Whole genome sequencing and antibiotic diffusion assays, provide new insight on drug resistance in the genus Pedobacter [J]. FEMS Microbiology Ecology, 2020, 96(6): 88-99.
Zhang H, Liu X, Gu Q, et al. Molecular characteristics and antibiotic resistance of Bacillus cereus from foods using whole genome sequencing [J]. Chinese Journal of Food Hygiene, 2021, (5): 529-535.
Zhang S, Yang G, Jiang Y. Antibiotic and metal resistance of Stenotrophomonas maltophilia isolates from Eboling permafrost of the Tibetan Plateau [J]. Environmental Science and Pollution Research, 2022, 30: 11798–117810.
Zhao G, Luo Z, Wang Y, et al. Draft genome sequencing and annotation of a low-virulence Morganella morganii strain CQ-M7, a multidrug-resistant isolate from the giant salamander in China [J]. The Journal of Global Antimicrobial Resistance, 2020, 20: 248-252.
Zhou Y, Zhong Z, Hu S, et al. A survey of helicobacter pylori antibiotic-resistant genotypes and strain lineages by whole-genome sequencing in china [J]. Antimicrobial Agents and Chemotherapy, 2022, 66(6): 2188-2203.
Costa K, Araujo F, Morais J, et al. Text mining for identification of biological entities related to antibiotic resistant organisms [J]. PeerJ, 2022, 10: 13351-13367.
Haffiez N, Chung T, Zakaria B, et al. Exploration of machine learning algorithms for predicting the changes in abundance of antibiotic resistance genes in anaerobic digestion [J]. Science of The Total Environment, 2022, 839: 156211-156224.
Her H, Wu Y. A pan-genome-based machine learning approach for predicting antimicrobial resistance activities of the Escherichia coli strains [J]. Bioinformatics, 2018, 34(13): 89-95.
Iftikhar S, Karim A, et al. Prediction and interpretation of antibiotic-resistance genes occurrence at recreational beaches using machine learning models [J]. Journal of Environmental Management, 2023, 328: 116969-116979.
Lamping F, Jack T, Ruebsamen N, et al. Development and validation of a diagnostic model for early differentiation of sepsis and non-infectious SIRS in critically ill children a data-driven approach using machine-learning algorithms [J]. BMC Pediatrics, 2018, 18: 112-123.
Mahe P, Tournoud M. Predicting bacterial resistance from whole-genome sequences using k-mers and stability selection [J]. BMC Biotechnology, 2018, 19: 383-394.
Rawson T, Hernandez B, WILSON R, et al. Supervised machine learning to support the diagnosis of bacterial infection in the context of COVID-19 [J]. JAC-Antimicrobal Resistance, 2021, 3(1): 2-6.
Sabiha S, Anuradha S, Arya S, et al. Genome informatics and machine learning-based identification of antimicrobial resistance-encoding features and virulence attributes in Escherichia coli genomes representing globally prevalent lineages, including high-risk clonal complexes [J]. Mbio, 2022, 13(1): 3796-3813.
Xu X, Liu S, Zeng M, et al. Deciphering response effect and underlying mechanism of anammox-based nitrogen removal process under exposures to different antibiotics via big data analysis [J]. Bioresource Technology, 2022, 347: 126674-126684.
Yang M, Wu Y. Enhancing predictions of antimicrobial resistance of pathogens by expanding the potential resistance gene repertoire using a pan-genome-based feature selection approach [J]. BMC Biotechnology, 2022, 23(4): 131-146.
Hiller C, Huebner U, Fajnorova S, et al. Antibiotic microbial resistance (AMR) removal efficiencies by conventional and advanced wastewater treatment processes: A review [J]. Science of the Total Environment, 2019, 685: 596-608.
Zhang J, Buhe C, Yu D, et al. Ammonia stress reduces antibiotic efflux but enriches horizontal gene transfer of antibiotic resistance genes in anaerobic digestion[J]. Bioresource Technology, 2020, 295: 122191.
Liu T, Mao Y, Shi Y, et al. Start-up and bacterial community compositions of partial nitrification in moving bed biofilm reactor [J]. Applied Microbiology And Biotechnology, 2017, 101(6): 2563-2574.
SYang C, Wang L, Wang H, et al. Dynamics of antibiotic resistance genes and microbial community in shortcut nitrification-denitrification process under antibiotic stresses [J]. Environmental Science and Pollution Research, 2022, 29(31): 46848-44858.
Wang L, Yang C, Yang Z, et al. Shortcut nitrification and denitrification shed light on simultaneous removal of conventional contaminants and antibiotic resistance genes (ARGs)[J]. Journal of Environmental Chemical Engineering, 2022, 10(1): 106925.
Wang X, Huang J, Gao D. Effects of three storage conditions on the long-term storage and short-term reactivation performances of anammox granular sludge [J]. International Biodeterioration & Biodegradation, 2021, 164: 105310.
Lee Y J, Park M K, Park G S, et al. Complete genome sequence of the thermophilic bacterium Geobacillus subterraneus KCTC 3922(T) as a potential denitrifier [J]. Journal of Biotechnology, 2017, 251: 141-144.
Tao X, Zhou A, Kempher M, et al. Development of a Markerless Deletion Mutagenesis System in Nitrate-Reducing Bacterium Rhodanobacter denitrificans [J]. Applied and Environmental Microbiology, 2022, 88(14): 1-13.
Awolusi O, Nasr M, Kumari S, et al. Artificial Intelligence for the Evaluation of Operational Parameters Influencing Nitrification and Nitrifiers in an Activated Sludge Process [J]. Microbial Ecology, 2016, 72(1): 49-63.
Forde B, Oliveira D, Falconer C, et al. Strengths and caveats of identifying resistance genes from whole genome sequencing data [J]. Expert Review Of Anti-infective Therapy, 2022, 20(4): 533-547.
Downloads
Published
Issue
Section
License
Copyright (c) 2024 韩 爱萍

This work is licensed under a Creative Commons Attribution 4.0 International License.






